Eloxx Pharmaceuticals Reports Additional Confirmation That All Nonsense Mutation Alport Syndrome Patients Treated With ELX-02 In Phase 2 Study Had Improvement In Kidney Morphology And Clinical Benefit Of Reduction Or Stabilization Of Proteinuria
Author: Benzinga Newsdesk | October 09, 2023 08:02am
ELX-02 treatment improved podocyte foot process effacement in all three patients by an average of 60% based on a blinded kidney biopsy analysis by NIPOKA GmbH
Biopsy results support clinical benefit in all three patients as improvement of kidney morphology is consistent with reduction or stabilization of proteinuria during or up to 2 months post completion of dosing
Renowned key opinion leaders recommend continued development following review of clinical and biopsy results
WATERTOWN, Mass., Oct. 09, 2023 (GLOBE NEWSWIRE) -- Eloxx Pharmaceuticals, Inc. (NASDAQ:ELOX), a leader in ribosomal RNA-targeted genetic therapies for rare diseases, today reported results from an assessment of patient biopsies by NIPOKA GmbH (Nipoka). They have developed a highly accurate method for the quantification of podocyte foot process morphology. These results confirm previously reported positive biopsy results from the proof-of-concept Phase 2 open-label clinical trial (NCT05448755) of ELX-02 for the treatment of Nonsense Mutation Alport syndrome patients. Analysis of formalin-fixed paraffin-embedded (FFPE) biopsy samples by Nipoka show ELX-02 treatment improved podocyte foot process morphology with lower effacement in all three patients at the end of the 8-week study period.
"With this accurate analysis of the patient biopsies and quantification of changes, we now have unequivocal evidence of morphology and clinical improvement in all three Nonsense Mutation Alport patients treated with ELX-02. Improvement in kidney morphology drives clinical benefit in this devastating rare disease," said Sumit Aggarwal, President and Chief Executive Officer of Eloxx. "We believe that our proteinuria data, during and after treatment, in the context of this improvement in kidney morphology, confirms clinical benefit in all three patients."
NIPOKA GmbH have developed a Podocyte Exact Morphology Procedure (PEMP) to quantify podocyte foot process morphology accurately and precisely in an unbiased and reproducible manner. PEMP utilizes immunostaining for foot-process specific protein markers followed by 3D-SIM imaging to quantify Filtration Slit Density (FSD) for 15 to 20 glomeruli per sample. FSD is a quantitative measure of the degree of podocyte foot process effacement. Higher FSD correlates with better podocyte health and lower podocyte foot process effacement. Healthy patients have an FSD of approximately 3.0 to 4.0. This analysis has been validated in multiple glomerular diseases.
PEMP analysis confirmed that ELX-02 treatment improved podocyte foot process effacement in all three patients with an average post-treatment increase in FSD of 60% as compared to baseline levels. These findings are also consistent with previous Transmission Electron Micrograph (TEM) image assessments.
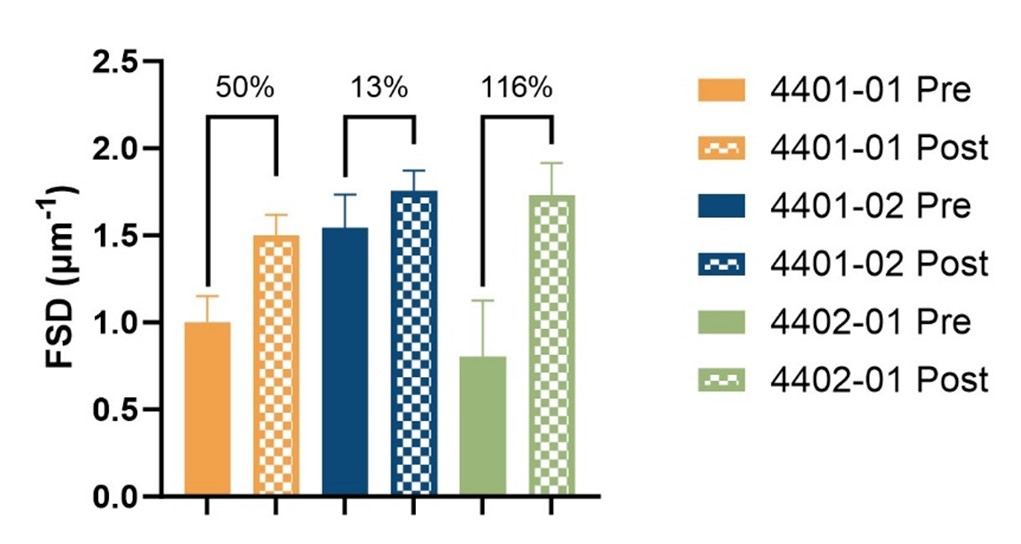
Differences in Urine Protein-Creatinine ratio (UPCR) changes across patients during treatment were correlated to severity of disease (lower vs. higher FSD) at baseline. Therefore, improvement in UPCR was assessed both during and 2 months after treatment to evaluate clinical benefit and capture the full effect of the 45-day protein half-life.
| Patient |
FSD at end
of treatment
(% change
vs. baseline) |
Average
change in
UPCR
during
treatment
vs. baseline |
Average change in
UPCR 2 months
after end of
treatment vs
baseline |
UPCR variability
change vs baseline
(Standard deviation
2 months after end
of treatment vs
baseline)
|
| 4401 – 01 |
1.50 (50%) |
No change |
No Change |
-32% |
| 4401 – 02 |
1.75 (13%) |
-49%; |
No Change |
-46% |
| 4402 – 01 |
1.73 (118%) |
No change |
-25% |
-68% |
As shown in the table above, all patients had proteinuria stabilization (lower variability vs. baseline) or improvement (reduction during or 2-months after treatment). This is consistent with clinical benefit and with the improvement in kidney morphology.
Renowned key opinion leaders have reviewed these data and overwhelmingly believe that they provide strong evidence of the potential of the disease modifying effect of ELX-02 and warrant advancement into a pivotal trial.
Posted In: ELOX




