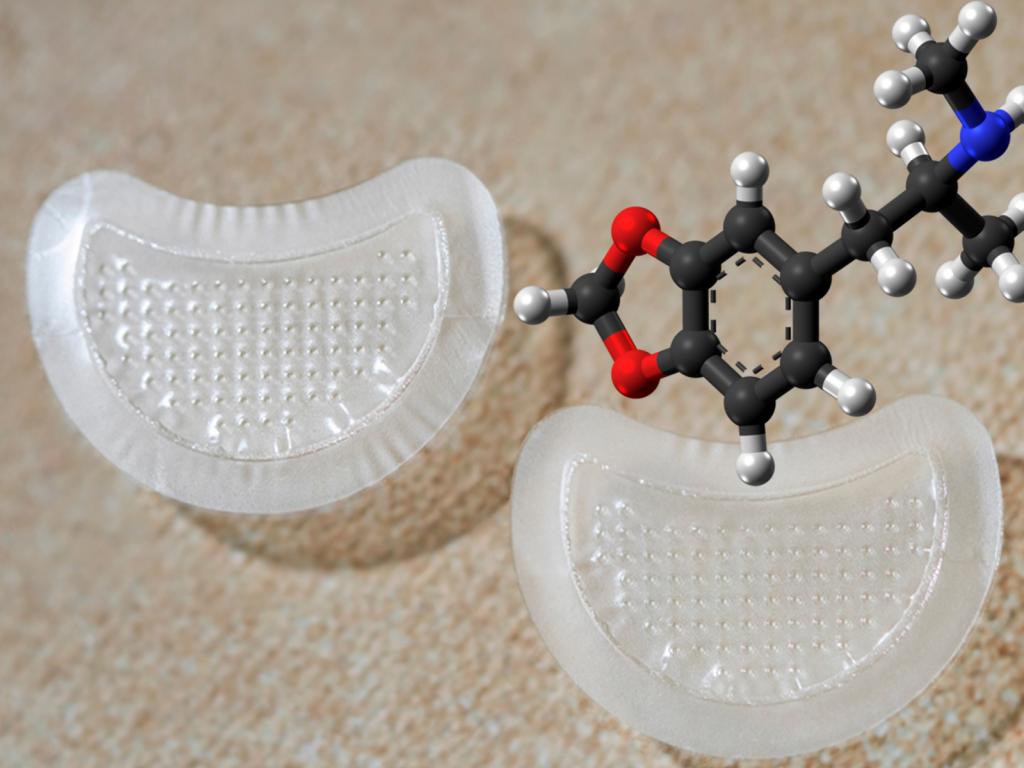
PharmaTher's DMT Patch Shows Positive Outcomes, Investment Toward The Clinical Stage
Author: Lara Goldstein | July 18, 2023 05:45pm
PharmaTher Holdings (OTCQB:PHRRF) and the Terasaki Institute for Biomedical Innovation evaluated the former’s novel microneedle patch for the delivery of DMT, and research yielded positive outcomes.
The study sought to develop a suitable prototype of PharmaPatch for DMT’s transdermal delivery to overcome obstacles associated with oral and IV administration routes.
Research provided a full characterization of DMT conjugated on the microneedle patch backbone, establishment and demonstration of the loading capacity for DMT, and release rate evaluations for the psychedelic-conjugated materials.
DMT microneedles have reportedly been “successfully fabricated, optimized, and characterized,” and demonstrated “acceptable performance” in male and female rats.
The authors state that the smooth incorporation of DMT into microneedles along with the demonstrated complete ex-vivo skin model release over several days hints at the potential for larger doses, modified controlled-release profiles, and microdosing.
Entering The Clinic
PharmaPatch’s delivery technology is reportedly based on novel biocompatible and biodegradable gelatin methacryloyl (GelMA) material to deliver water-soluble and insoluble drugs with specified release profiles in a safe and efficient manner by penetrating the outer layer of the skin.
PharmaTher completed research studies demonstrating that its microneedle patch can deliver other psychedelics including psilocybin, MDMA and LSD, and believes PharmaPatch’s achieved prototype is acceptable for completing IND-enabling studies toward entering clinical studies next.
The company will work with its research partner PharmaDrug Inc., as well as invest $227,700 (CA$300,000) in the latter’s subsidiary, Sairiyo Therapeutics, for a 49% ownership.
Sairiyo is developing novel uses and delivery forms of DMT and other undisclosed tryptamines as a potential treatment for ocular disease and neuropsychiatric conditions.
In connection with the investment, PharmaDrug and PharmaTher will enter into a unanimous shareholders agreement regarding their Sairiyo holdings, concerning management appointment rights and the constitution of the board of directors and shareholders' entitlement to subscribe for new share issuances on a pro-rata basis.
Wondering about the latest in the cannabis business world? Get ahead by attending Benzinga’s Cannabis Capital Conference!
Taking place in Chicago on Sept 27-28, the 17th edition of CCC is the place to get DEALS DONE. Join us by getting your tickets today before prices increase.
Photo: Benzinga edit with photo by DyrElena by Shutterstock and Wikimedia Commons.
Posted In: PHRRF